proteostasis in health and disease
The functional state of a cell is ultimately defined by the state of its proteome, i.e. abundance, localisation, turnover and mobility of all proteins and their organisation in complexes and organelles. Numerous cellular systems contribute to proteome homeostasis through prevention, detection and removal of abnormal proteins. Selective protein degradation by the ubiquitin-proteasome systems plays a key role in proteome turnover and quality control. When degradation is not possible, the impact of abnormal proteins can be minimised through their asymmetric partitioning during cell division. Despite the activity of such systems, proteome homeostasis declines with aging and in numerous diseases, resulting in accumulation of abnormal proteins and loss of cell functionality.
We want to understand how cells deal with abnormal proteins. For that, we aim to systematically identify substrates for the various components of the ubiquitin-proteasome system and explore the functions of this system in replicative ageing and genome stability. We are using genetic and proteomic approaches that exploit fluorescent timers to follow protein trafficking, inheritance and degradation down to subcellular resolution. Our goals are to understand how protein quality control is coordinated with protein synthesis, folding and trafficking, to elucidate how cells recognise abnormal proteins, and how they adapt to challenges in proteome homeostasis.
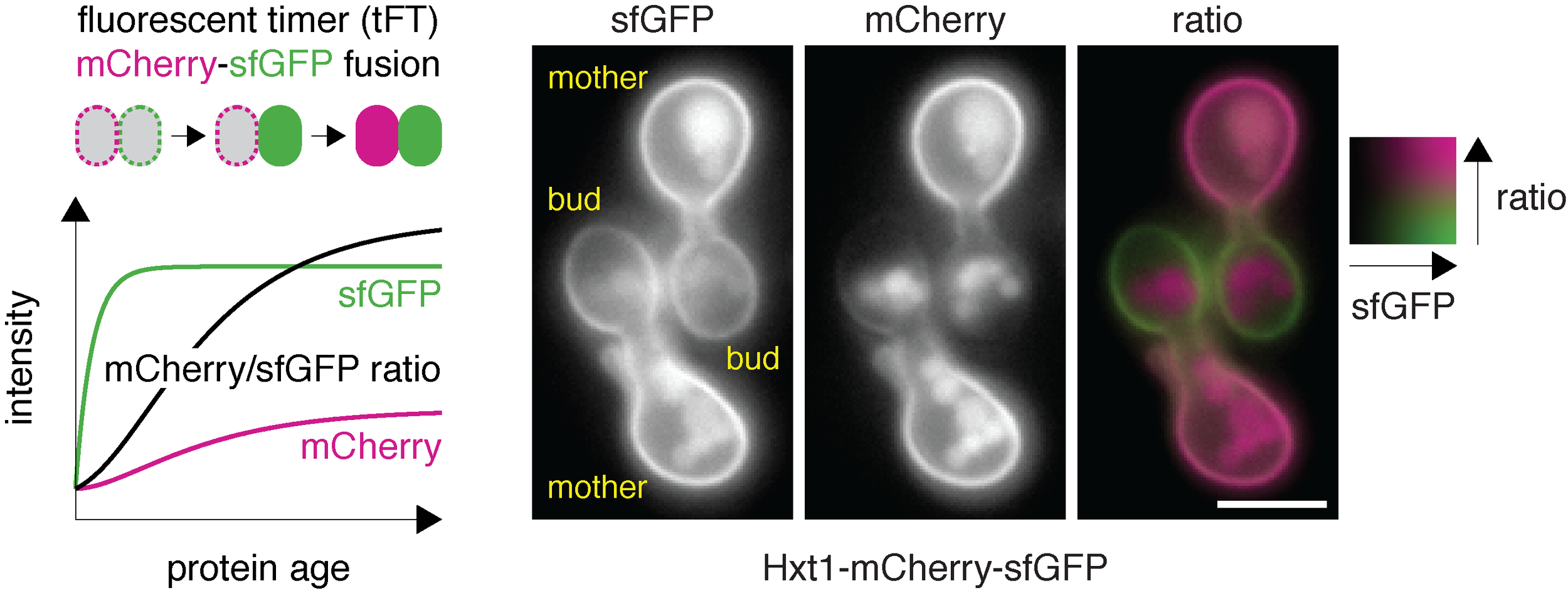
fluorescent timers
A tandem fluorescent protein timer (tFT) is a fusion of two conventional fluorescent proteins: for instance sfGFP (green) and mCherry (red). Due to different maturation kinetics of mCherry (slow) and sfGFP (fast), the mCherry/sfGFP ratio is a measure of protein age and can be used to follow trafficking, degradation and inheritance (as in this example of the Hxt1 glucose transporter) of tFT-tagged proteins.
protein stability arrays
High-throughput measurements of protein turnover with tFTs. Yeast strains expressing tFT-tagged proteins were grown as colony arrays on agar medium. Colony fluorescence was recorded with a fluorescence plate reader.